16 mei 2022: Bron: Nature, Communications Medicine volume 2, Article number: 29 (2022)
Een nieuwe techniek in het opsporen van alvleesklierkanker in een vroeg stadium (Stadium I en II) blijkt bijzonder succesvol. Een bloedtest gebaseerd op een eiwit dat biomarkers meet in zogeheten extracellulaire blaasjes (EV's) ontdekte 95 procent van beginnende alvleeskliertumoren. Bij eierstokkanker en blaaskanker waren de percentages minder, respectievelijk 74 procent en 43 procent, maar ook deze vormen van kanker worden vaak pas ontdekt als het al uitgezaaid is.
Alvleesklierkanker is een van de dodelijkste kankers. Het wordt zelden gediagnosticeerd voordat het zich begint te verspreiden en heeft een overlevingspercentage van minder dan 5% over een periode van vijf jaar. Aan de University of California San Diego School of Medicine hebben wetenschappers nu een test ontwikkeld die 95% van de vroege alvleesklierkankers in een onderzoek kon identificeren.
Het onderzoek, gepubliceerd in Nature Communications Medicine, legt uit hoe biomarkers in extracellulaire blaasjes – deeltjes die de communicatie tussen cellen reguleren – werden gebruikt om alvleesklierkanker, eierstokkanker en blaaskanker op te sporen in de stadia I en II.
"Alvleesklierkanker is bijzonder moeilijk om vroeg te detecteren, in een stadium waarin chirurgische resectie - operatie de enige genezende behandeling mogelijk is.", zo zegt Dr. Andrew Lowy, clinical director for Cancer Surgery at UC San Diego School of Medicine. De mediane overall overleving op 5 jaar is voor alvleesklierkanker de laagste van alle vormen van kanker.
In de afgelopen decennia hebben kankeronderzoekers tientallen aan kanker gerelateerde biomarkers ontdekt die een rol spelen bij de groei en overleving van kanker. Deze ontdekkingen hebben geleid tot de ontwikkeling van effectieve medicijnen tegen kanker. De onderzoekers van UCSD vermoedden dat deze moleculen kunnen worden gebruikt om kanker vroeg te identificeren. Helaas zijn deze moleculen heel lastig te vinden.
Multi-kankerdetectietests (MCDT's) omvatten screening op bloedgebaseerde eiwitten of nucleïnezuren die indicatief zijn voor kanker. Verschillende MCD-tests zijn veelbelovend gebleken voor het opsporen van kanker in een laat stadium. Het opsporen van kanker in een vroeg stadium is echter nog steeds een uitdaging.
Tijdens de vroege stadia van kwaadaardige tumoren zijn er zeer weinig kankergerelateerde biomarkers en veel niet-gerelateerde moleculen. Bijgevolg zijn Multi-kankerdetectietests (MCDT's) niet gevoelig genoeg om vroege tekenen van kanker te herkennen. Met andere woorden, er is teveel achtergrondgeluid.
In deze studie besloten de onderzoekers dat ze zich maar op één ding zouden concentreren: extracellulaire blaasjes – deeltjes die de communicatie tussen cellen reguleren.
Extracellulaire blaasjes (EV's) kunnen worden gebruikt om te screenen op vroege kankersignalen.
Gezonde cellen en kankercellen stoten EV's uit in de bloedbaan. Van kanker afgeleide EV's bevatten vaak veel kankergerelateerde eiwitbiomarkers, die problemen kunnen veroorzaken. Wanneer deze eiwitten aan andere kankercellen worden afgeleverd, kunnen ze de resistentie tegen chemotherapeutica verhogen, de metastase versterken, de afgifte van voedingsstoffen verhogen en het immuunsysteem verstoren, is aangetoond in eerdere studies. Maar zeggen de onderzoekers: de inhoud van extracellulaire blaasjes heeft diagnostisch potentieel.
Sommige van alvleesklierkanker afgeleide EV's dragen bijvoorbeeld een eiwit genaamd macrofaagremmende factor (MIF), dat het immuunsysteem onderdrukt. De hoeveelheid MIF in het blaasje kan echter dienen als een voorspellende marker voor een leveruitzaaiing. Dat wil zeggen, hoe meer MIF er is, hoe groter de kans dat de kanker zich naar de lever zal verspreiden.
Lowy en zijn team zuiverden EV's uit het bloed van patiënten met vroege alvleesklierkanker, eierstokkanker en blaaskanker, en daarnaast van gezonde controle patiënten.
Vervolgens analyseerden ze de eiwitsamenstelling van de monsters. Door de monsters van kanker- en controlepatiënten te vergelijken, ontwikkelden de wetenschappers een machinaal lerend algoritme om een kleine set EV-eiwitten te identificeren die kunnen worden gebruikt om alvleesklierkanker, eierstokkanker en blaaskanker in een vroeg stadium te detecteren.
Hun algoritme detecteerde met succes 95,5% van stadium I alvleesklierkanker, 73,1% van stadium I eierstokkanker en 43,8% van stadium I blaaskanker, wat de potentiële waarde van deze technologie voor vroege opsporing van kanker illustreert.
In onderstaande grafiek de gevoeligheid van de test bij de drie vormen van kanker.
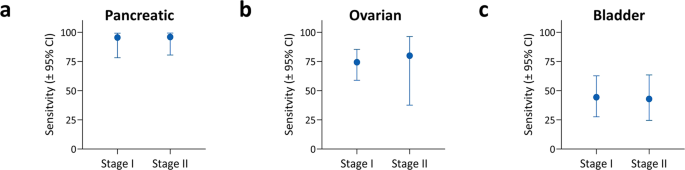
a Sensitivity for detecting either stage I (N = 22) or stage II (N = 25) pancreatic cancer. b Sensitivity for detecting either stage I (N = 39) or stage II (N = 5) ovarian cancer. c Sensitivity for detecting either stage I (N = 27) or stage II (N = 21) bladder cancer. All sensitivities represent values at >99% specificity for the held-out test sets. Error bars in all panels represent the two-sided 95% Wilson confidence intervals.
Het studierapport is gratis in te zien. Klik op de titel van het abstract:
- Article
- Open Access
- Published:
Early-stage multi-cancer detection using an extracellular vesicle protein-based blood test
Communications Medicine volume 2, Article number: 29 (2022)
Abstract
Background
Detecting cancer at early stages significantly increases patient survival rates. Because lethal solid tumors often produce few symptoms before progressing to advanced, metastatic disease, diagnosis frequently occurs when surgical resection is no longer curative. One promising approach to detect early-stage, curable cancers uses biomarkers present in circulating extracellular vesicles (EVs). To explore the feasibility of this approach, we developed an EV-based blood biomarker classifier from EV protein profiles to detect stages I and II pancreatic, ovarian, and bladder cancer.
Methods
Utilizing an alternating current electrokinetics (ACE) platform to purify EVs from plasma, we use multi-marker EV-protein measurements to develop a machine learning algorithm that can discriminate cancer cases from controls. The ACE isolation method requires small sample volumes, and the streamlined process permits integration into high-throughput workflows.
Results
In this case-control pilot study, comparison of 139 pathologically confirmed stage I and II cancer cases representing pancreatic, ovarian, or bladder patients against 184 control subjects yields an area under the curve (AUC) of 0.95 (95% CI: 0.92 to 0.97), with sensitivity of 71.2% (95% CI: 63.2 to 78.1) at 99.5% (97.0 to 99.9) specificity. Sensitivity is similar at both early stages [stage I: 70.5% (60.2 to 79.0) and stage II: 72.5% (59.1 to 82.9)]. Detection of stage I cancer reaches 95.5% in pancreatic, 74.4% in ovarian (73.1% in Stage IA) and 43.8% in bladder cancer.
Conclusions
This work demonstrates that an EV-based, multi-cancer test has potential clinical value for early cancer detection and warrants future expanded studies involving prospective cohorts with multi-year follow-up.
Plain Language Summary
Finding cancer early can make treatment easier and improve odds of survival. However, many tumors go unnoticed until they have grown large enough to cause symptoms. While scans can detect tumors earlier, routine full-body imaging is impractical for population screening. New cancer detection methods being explored are based on observations that tumors release tiny particles called extracellular vesicles (EVs) into the bloodstream, containing proteins from the tumor. Here, we used a method to purify EVs from patients’ blood followed by a method to detect tumor proteins in the EVs. Our method quickly and accurately detected early-stage pancreatic, ovarian, or bladder cancer. With further testing, this method may provide a useful screening tool for clinicians to detect cancers at an earlier stage.
References
-
Heitzer, E., Perakis, S., Geigl, J. B. & Speicher, M. R. The potential of liquid biopsies for the early detection of cancer. npj Precis. Oncol. 1, 36 (2017).
-
Blackford, A. L., Canto, M. I., Klein, A. P., Hruban, R. H. & Goggins, M. Recent trends in the incidence and survival of stage 1A pancreatic cancer: a surveillance, epidemiology, and end results analysis. J. Natl Cancer Inst. 112, 1162–1169 (2020).
-
Muralidhar, V. et al. Association between very small tumor size and decreased overall survival in node-positive pancreatic cancer. Ann. Surg. Oncol. 25, 4027–4034 (2018).
-
Stewart, C., Ralyea, C. & Lockwood, S. Ovarian cancer: an integrated review. Semin. Oncol. Nurs. 35, 151–156 (2019).
-
Siegel, R. L., Miller, K. D., Fuchs, H. E. & Jemal, A. Cancer statistics, 2021. CA 71, 7–33 (2021).
-
Surveillance Research Program, N. C. I. SEER*Explorer: An interactive website for SEER cancer statistics, <https://seer.cancer.gov/data-software/> (2021).
-
Lenis, A. T., Lec, P. M., Chamie, K. & MSHS, M. Bladder cancer: a review. JAMA 324, 1980–1991 (2020).
-
Smith, R. A. & Oeffinger, K. C. The importance of cancer screening. Med. Clin. North. Am. 104, 919–938 (2020).
-
Mader, S. & Pantel, K. Liquid biopsy: current status and future perspectives. Oncol. Res. Treat. 40, 404–408 (2017).
-
Shen, S. Y. et al. Sensitive tumour detection and classification using plasma cell-free DNA methylomes. Nature 563, 579–583 (2018).
-
Phallen, J. et al. Direct detection of early-stage cancers using circulating tumor DNA. Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.aan2415 (2017).
-
Liu, M. C. et al. Sensitive and specific multi-cancer detection and localization using methylation signatures in cell-free DNA. Ann. Oncol. 31, 745–759 (2020).
-
Klein, E. A. et al. Clinical validation of a targeted methylation-based multi-cancer early detection test using an independent validation set. Ann. Oncol. 32, 1167–1177 (2021).
-
Cohen, J. D. et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science 359, 926–930 (2018).
-
Blume, J. E. et al. Rapid, deep and precise profiling of the plasma proteome with multi-nanoparticle protein corona. Nat. Commun. 11, 3662 (2020).
-
Chen, X. et al. Prognostic significance of blood-based multi-cancer detection in plasma cell-free DNA. Clin. Cancer Res. 27, 4221–4229 (2021).
-
Zhou, B. et al. Application of exosomes as liquid biopsy in clinical diagnosis. Signal Transduct. Target. Therapy 5, 144 (2020).
-
Kalluri, R. & LeBleu, V. S. The biology, function, and biomedical applications of exosomes. Science 367, eaau6977 (2020).
-
Hoshino, A. et al. Tumour exosome integrins determine organotropic metastasis. Nature 527, 329–335 (2015).
-
Yu, W. et al. Exosome-based liquid biopsies in cancer: opportunities and challenges. Ann. Oncol. 32, 466–477 (2021).
-
Hoshino, A. et al. Extracellular vesicle and particle biomarkers define multiple human cancers. Cell 182, 1044–1061.e1018 (2020).
-
Min, L. et al. Advanced nanotechnologies for extracellular vesicle-based liquid biopsy. Adv. Sci. https://doi.org/10.1002/advs.202102789 (2021).
-
Hinestrosa, J. P. et al. Simultaneous isolation of circulating nucleic acids and EV-associated protein biomarkers from unprocessed plasma using an AC electrokinetics-based platform. Front. Bioeng. Biotechnol. https://doi.org/10.3389/fbioe.2020.581157 (2020).
-
Ibsen, S. D. et al. Rapid isolation and detection of exosomes and associated biomarkers from plasma. ACS Nano 11, 6641–6651 (2017).
-
Ibsen, S. et al. Nanoparticles: recovery of drug delivery nanoparticles from human plasma using an electrokinetic platform technology. Small 11, 4990–4990 (2015).
-
Sonnenberg, A. et al. Dielectrophoretic isolation and detection of cancer-related circulating cell-free DNA biomarkers from blood and plasma. Electrophoresis 35, 1828–1836 (2014).
-
Manouchehri, S. et al. Dielectrophoretic recovery of DNA from plasma for the identification of chronic lymphocytic leukemia point mutations. Int. J. Hematol. Oncol. 5, 27–35 (2016).
-
Lewis, J. M. et al. Integrated analysis of exosomal protein biomarkers on alternating current electrokinetic chips enables rapid detection of pancreatic cancer in patient blood. ACS Nano 12, 3311–3320 (2018).
-
Lewis, J. et al. A pilot proof-of-principle analysis demonstrating dielectrophoresis (DEP) as a glioblastoma biomarker platform. Sci. Rep. 9, 10279 (2019).
-
Liu, L. et al. Machine learning protocols in early cancer detection based on liquid biopsy: a survey. Life (Basel) https://doi.org/10.3390/life11070638 (2021).
-
McClish, D. K. Analyzing a portion of the ROC curve. Med. Decis. Making 9, 190–195 (1989).
-
Michiels, S., Koscielny, S. & Hill, C. Prediction of cancer outcome with microarrays: a multiple random validation strategy. Lancet 365, 488–492 (2005).
-
Baker, S. G. & Kramer, B. S. Identifying genes that contribute most to good classification in microarrays. BMC Bioinformatics 7, 407 (2006).
-
Ambroise, C. & McLachlan, G. J. Selection bias in gene extraction on the basis of microarray gene-expression data. Proc. Natl Acad. Sci. USA 99, 6562–6566 (2002).
-
Efron, B. Nonparametric standard errors and confidence intervals. Can. J. Stat./ La Revue Canadienne de Statistique 9, 139–158 (1981).
-
Brown, L. D., Cai, T. T. & DasGupta, A. Interval estimation for a binomial proportion. Stat. Sci. 16, 101–117 (2001).
-
Théry, C. et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 7, 1535750 (2018).
-
Patel, G. K. et al. Comparative analysis of exosome isolation methods using culture supernatant for optimum yield, purity and downstream applications. Sci. Rep. 9, 5335 (2019).
-
Mol, E. A., Goumans, M. J., Doevendans, P. A., Sluijter, J. P. G. & Vader, P. Higher functionality of extracellular vesicles isolated using size-exclusion chromatography compared to ultracentrifugation. Nanomedicine 13, 2061–2065 (2017).
-
Hastie, T., Tibshirani, R. & Friedman, J. H. The Elements of Statistical Learning: Data Mining, Inference, and Prediction. (Springer, 2009).
-
Niland, S. & Eble, J. A. Neuropilins in the context of tumor vasculature. Int. J. Mol. Sci. 20, 639 (2019).
-
Kufe, D. W. MUC1-C oncoprotein as a target in breast cancer: activation of signaling pathways and therapeutic approaches. Oncogene 32, 1073–1081 (2013).
-
Miao, H.-Q., Lee, P., Lin, H., Soker, S. & Klagsbrun, M. Neuropilin-1 expression by tumor cells promotes tumor angiogenesis and progression. FASEB J. 14, 2532–2539 (2000).
-
Kjaergaard, A. G., Dige, A., Nielsen, J. S., Tønnesen, E. & Krog, J. The use of the soluble adhesion molecules sE-selectin, sICAM-1, sVCAM-1, sPECAM-1 and their ligands CD11a and CD49d as diagnostic and prognostic biomarkers in septic and critically ill non-septic ICU patients. Apmis 124, 846–855 (2016).
-
Pranjol, M. Z. I., Gutowski, N., Hannemann, M. & Whatmore, J. The potential role of the proteases Cathepsin D and Cathepsin L in the progression and metastasis of epithelial ovarian cancer. Biomolecules 5, 3260–3279 (2015).
-
Lee, S., Jeon, H. & Shim, B. Prognostic value of ferritin-to-hemoglobin ratio in patients with advanced non-small-cell lung cancer. J. Cancer 10, 1717–1725 (2019).
-
El Fitori, J. et al. Melanoma Inhibitory Activity (MIA) increases the invasiveness of pancreatic cancer cells. Cancer Cell Int. 5, 3 (2005).
-
Baxter, R. C. IGF binding proteins in cancer: mechanistic and clinical insights. Nat. Rev. Cancer 14, 329–341 (2014).
-
Peter, M. E. et al. The role of CD95 and CD95 ligand in cancer. Cell Death Differ. 22, 549–559 (2015).
-
Liu, N. et al. Human chorionic gonadotropin β regulates epithelial-mesenchymal transition and metastasis in human ovarian cancer. Oncol. Rep. 38, 1464–1472 (2017).
-
Suwinski, R. et al. Blood serum proteins as biomarkers for prediction of survival, locoregional control and distant metastasis rate in radiotherapy and radio-chemotherapy for non-small cell lung cancer. BMC Cancer 19, 427 (2019).
-
Skogberg, G. et al. Characterization of human thymic exosomes. PLoS ONE 8, e67554 (2013).
-
Liang, B. et al. Characterization and proteomic analysis of ovarian cancer-derived exosomes. J. Proteom. 80, 171–182 (2013).
-
Guo, X.-Y. et al. Exosomes and pancreatic diseases: status, challenges, and hopes. Int. J. Biol. Sci. 15, 1846–1860 (2019).
-
Chavez-Muñoz, C., Kilani, R. T. & Ghahary, A. Profile of exosomes related proteins released by differentiated and undifferentiated human keratinocytes. J. Cell. Physiol. 221, 221–231 (2009).
-
Charkhchi, P. et al. CA125 and ovarian cancer: a comprehensive review. Cancers 12, 3730 (2020).
-
Kim, S. et al. Carbohydrate antigen 19-9 elevation without evidence of malignant or pancreatobiliary diseases. Sci. Rep. 10, 8820 (2020).
-
Warrick, J. I. et al. Intratumoral heterogeneity of bladder cancer by molecular subtypes and histologic variants. Eur. Urol. 75, 18–22 (2019).
-
Kang, H. W., Kim, W. J., Choi, W. & Yun, S. J. Tumor heterogeneity in muscle-invasive bladder cancer. Transl. Androl. Urol. 9, 2866–2880 (2020).
-
Fane, M. & Weeraratna, A. T. How the ageing microenvironment influences tumour progression. Nat. Rev. Cancer 20, 89–106 (2020).
Acknowledgements
We are grateful to Proteogenex for their support in collecting clinical samples for this study, Drs. Alex Aleshin and Varia Kirchner for their input during the development of this study, and Drs. Eric Varma and Irwin Jacobs for their continued support of Biological Dynamics and advice on this study and manuscript preparation. Zen Bio Incorporated (Research Triangle, NC) is acknowledged for help on the differential ultracentrifugation isolation. Figures 1 and 2 were created with the assistance from Biorender.com.
Author information
Affiliations
Contributions
R. Kurzrock, R. Krishnan, J.P.H., J.M.L., R.T., P.B., M.A., I.C., H.I.B., and S.M.L. designed the study, wrote, and revised the manuscript. J.P.H. and L.A. supervised the experimental work. J.P.H., V.O., O.P., and H.I.B. collected and organized samples for the cohort. J.M.L., D.S., O.P., K.R., J.R.H., L.A., J.P.H., and S.M.L. performed or supervised experimental protocols. J.P.H., G.S., and N.J.S. analyzed the experimental data and developed the classification model. R. Kurzrock, A.M.K., R.E., A.M.L., and S.M.L. reviewed clinical data. All authors approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
R. Krishnan, J.P.H., R.T., I.C., H.I.B., V.O., J.M.L., O.P., L.A., J.R.H., G.S., and D.S. are employees of Biological Dynamics. R. Krishnan is a co-founder and board member of Biological Dynamics. R. Krishnan is an inventor on patents held by the University of California San Diego and Biological Dynamics that covers aspects of the Verita™ platform used in this manuscript. The terms of these arrangements are being managed by the University of California–San Diego in accordance with its conflict-of-interest policies. R. Kurzrock receives research funding from Boehringer Ingelheim, Debiopharm, Foundation Medicine, Genentech, Grifols, Guardant, Incyte, Konica Minolta, Medimmune, Merck Serono, Omniseq, Pfizer, Sequenom, Takeda, and TopAlliance; as well as consultant and/or speaker fees and/or advisory board for Actuate Therapeutics, Bicara Therapeutics, Inc., Biological Dynamics, Neomed, Pfizer, Roche, TD2/Volastra, Turning Point Therapeutics, X-Biotech; has an equity interest in CureMatch Inc. and ID by DNA; serves on the Board of CureMatch and CureMetrix, and is a co-founder of CureMatch. R.E. receives research funding to his institution from Clovis Oncology, AVITA, Merck and AstraZenca, as well as consultant and/or speaker fees and/or advisory board from AstraZeneca, GSK/Tesaro, Seagen, Myriad, Merck, Eisai as well as the GOG Foundation. A.K. consultant/advisory board member for Abbott Molecular, Arquer, ArTara, Asieris, Astra Zeneca, BioClin Therapeutics, Biological Dynamics, BMS, Cepheid, Cold Genesys, Eisai, Engene, Inc., Ferring, FerGene, Imagin, Janssen, MDxHealth, Medac, Merck, Pfizer, Photocure, ProTara, Roviant, Seattle Genetics, Sessen Bio, Theralase, TMC Innovation, US Biotest. AM Kamat has received grant/research support from Adolor, BMS, FKD Industries, Heat Biologics, Merck, Photocure, SWOG/NIH, SPORE, AIBCCR. A.M.K. has patents for CyPRIT (Cytokine Predictors of Response to Intravesical Therapy) jointly with UT MD Anderson Cancer Center is a paid consultant of Biological Dynamics. S.M.L. is a co-founder of io9. N.J.S., S.M.L., P.B., and M.A. are members of the Biological Dynamics scientific advisory board. S.M.L. received principal investigator support from the UC San Diego Moores Cancer Center, Specialized Cancer Center Support Grant NIH/NCI P30CA023100, and SU2C-AACR-DT-25-17 Pancreatic Cancer Interception Dream Team award. A.M.L. and R.E. declare no competing interests. P.B. holds equity in CytoBay, Synergenz, and LungLifeAI, all cancer diagnostic or risk assessment enterprises.
Peer review
Peer review information
Communications Medicine thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
Gerelateerde artikelen
- Vloeibare biopsie gericht op exosomen (tumorblaasjes) registreerde 97 procent van de patienten met alvleesklierkanker stadium 1 en 2 in combinatie met de biomarker CA 19-9 van gezonde mensen
- Bloedtest gebaseerd op een eiwit circulerend in extracellulaire blaasjes ontdekt 95 procent van beginnende alvleesklierkanker in vroeg stadium, 74 procent bij eierstokkanker en 43 procent bij blaaskanker.
- Urinetest meet lagere waarden van calcium en magnesium en verhoogde waarden van koper en zink bij alvleesklierkankerpatienten in vergelijking met gezonde mensen
- Een jaarlijks screeningprogramma bij mensen met hoog risico op alvleesklierkanker ontdekt relatief veel vroegtijdig (pre-) kwaadaardige tumoren.
- Biliscreen, een speciale app kan via een selfie van oog vroegtijdig alvleesklierkanker en aan geelzucht gerelateerde leveraandoeningen ontdekken. copy 1
- Diagnose: Sterk verhoogde CA19-9 waarde duidt op inoperabele alvleesklierkanker en is diagnostisch beter dan kijkoperaties en voorkomt daarmee onnodige belastende en dure operatieve ingrepen.
- Diagnose van alvleesklierkanker, een overzicht



Plaats een reactie ...
Reageer op "Bloedtest gebaseerd op een eiwit circulerend in extracellulaire blaasjes ontdekt 95 procent van beginnende alvleesklierkanker in vroeg stadium, 74 procent bij eierstokkanker en 43 procent bij blaaskanker."